THE IMMUNE SYSTEM
The immune system is as complex as the brain, but this oversimplification will illustrate what might go wrong in opsoclonus-myoclonus.
What is the immune system?
The body's innate defense against disease is natural immunity. The immune system consists of white blood cells, cell products, and other substances.
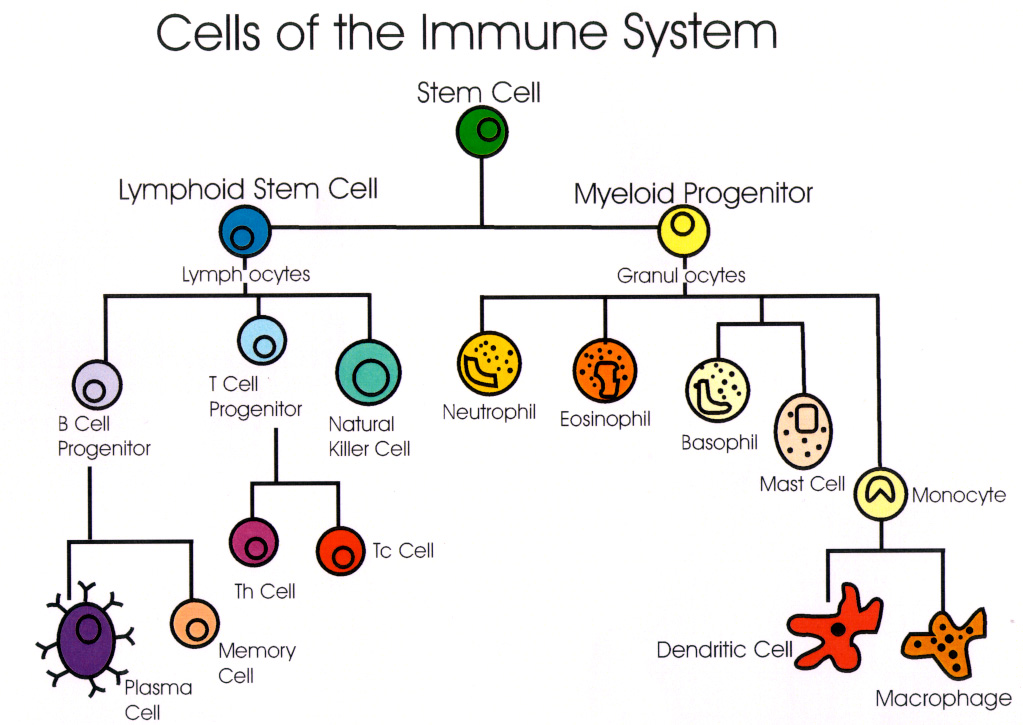
Lymphocytes include:
- T cells (T for thymus, where they mature)
- B cells (B for bone marrow, where they originate)
- Natural killer (NK) cells (less abundant)
Other white blood cells include monocytes-macrophages and granulocytes (neutrophils, eosinophils, and basophils). They are phagocytic or "cell-eating."
The fabric of the immune system is lymphoid tissue, which includes bone marrow, the thymus (a gland beneath the breastbone), lymph nodes, spleen, tonsils, gut and other mucosal areas.
Complement is a soluble protein secretion in blood that destroys pathogens when it is activated.
The cells of the immune system can be identified by their microscopic appearance and by surface CD markers, of which there are nearly two hundred.
How does the immune system know when to respond?
The immune system goes into a "search-out and destroy" mode when it "recognizes" foreign antigens. An antigen is any of an enormous range of substances that can be bound by an antibody and induce some kind of immune response.

The immune system's first encounter with an antigen is the primary response. Re-encounter with the same antigen causes a more powerful and rapid secondary response. This acquired immunity involves lymphocytes.
Activated lymphocytes divide and produce chemokines, the chemical signals between cells of the immune system. Some lymphocytes may never have been exposed to the antigen; others "remember" it. These cells are called "na´ve" (unprimed) cells and "memory" (primed) cells.
The main immune defenses against tumors are T cells, natural killer cells, and macrophages. Together with B cells and complement, they also play a role against infections.
Do B lymphocytes and T lymphocytes respond in the same way to antigens?
B cells and T cells "see" antigen differently. B cells recognize native antigen. T cells recognize processed antigen. What they have in common is that each cell recognizes only one very specific antigen.
B cells respond to antigens by producing antibodies (also called gamma globulins or immunoglobulins) that bind to the antigen. In this activated state they are called plasma cells. Antibodies are Y-shaped proteins. The antibodies of concern in opsoclonus-myoclonus are IgG and IgM.

In infections, IgM is first produced, then IgG appears weeks later. IgG is the most common antibody in blood. Each antibody has subtypes. Previous encounters with the same antigen produce memory B cells. Complement can bind to the antigen-antibody complex, but not all antibody responses involve complement.
Antibodies destroy the invader by blocking its activity (neutralization), allowing phagocytic white blood cells to recognize it (opsonisation), or by lysing the antigen-containing cell (complement activation).
Some antibody responses cannot occur without the help of certain T cells called helper cells (CD4). These cells help B cells produce antibody and help other T cells to kill target cells. However, viruses like EBV (the cause of mononucleosis), which can trigger opsoclonus-myoclonus, are capable of directly stimulating B cells to produce antibodies independently of T cells.

What's different about T cell responses?
T cells respond to antigens by producing cytokines, not antibodies. When activated, some T cells become cytotoxic (CD8) and kill tumor cells or infected cells. Cytokines that promote inflammation include certain interleukins, tumor necrosis factor, and interferon gamma. Other cytokines, like transforming growth factor, are immunosuppressants. So T cells may either enhance or suppress inflammation.

T cells only recognize antigen presented to them by other cells called antigen-presenting cells (APCs). In the blood, these are often macrophages or B cells; in the brain, microglia. Unlike natural killer cells, which have innate ability to recognize and destroy tumor cells or infected cells, T cells must follow a tightly regulated protocol involving the major histocompatibility complex (MHC I or II).
MHC proteins allow T cells to distinguish "self" from "non-self." When class II MHC proteins present antigens, they are detected by different T cells (T-helper) than when class I MHC proteins are involved (T-cytotoxic). Non-self antigens are targeted for destruction.
So the immune response to antigens has two components
- antibody production
- cell-mediated immunity
Can blood lymphocytes enter the brain?
Normally the brain is protected by a special anatomic defense, the blood-brain barrier. This privileged status is unique. Only a small percent of antibodies in the blood cross the barrier. Lymphocytes cannot enter unless they are activated. However, once activated, both T cells and B cells can search the brain for an antigen like the one that activated them. If they don't find it, they leave the brain and return to the blood. This kind of trafficking is routine.

However, if they do locate the antigen, they attack. Antibodies made by plasma cells attach to brain cells, block receptors, perforate the cell membrane, or otherwise injure or kill the cells. Cytotoxic T cells have the same lethal power.
What goes wrong in autoimmune disease?
As long as the immune system recognizes the body's own tissues as self, things are okay. But when it gets its signals crossed and interprets self as non-self, autoimmune disease may develop. One in 20 Americans has an autoimmune disease
With 50 to 100 million different circulating antibodies and millions of immune cells, why don't we all develop autoimmune disease? A balanced immune response is achieved by cell-to-cell contact and secretion of chemical factors. Multiple immune-response genes regulate how antigens are handled. During development, T cells that do not learn tolerance to the body's own proteins are destroyed. This huge immune "network" also has built-in checks and balances. Opposing types of T cells, such as helper/inducer cells and cytotoxic/suppressor cells, are usually kept in balance.
When the combination of environmental, hormonal, and genetic factors is unfavorable, autoimmune disease occurs. It may be triggered by exposure to cross-reacting antigens, impaired immunoregulation, or appearance of normally hidden antigens. Hidden brain antigens are normally prevented by the blood-brain barrier from leaking out to the blood, where they would be recognized as foreign and precipitate an immune response. An antigen may not stimulate an immune response unless co-stimulation has occurred.
The main co-stimuli that empower an immune response are:
- virus
- tumor
- vaccine
As an example of how one cell type can make a huge difference, when T-helper cell numbers plummet in AIDS, the body is left wide open to infections that healthy persons would have no trouble resisting. When T-helper activity is abnormally increased, a disease like multiple sclerosis may result. In normal individuals, T cells don't go uncontrolled because every cell has a built-in suicide mechanism (apoptosis).
How does the immune system fail in opsoclonus-myoclonus?
We believe the immune system fails twice. When neuroblastoma is the cause, the immune system allows the tumor to grow, then seems to overreact, and in the process injures the brain.
Such confusion could happen in a number of ways. Tumor or viral antigens may resemble brain antigens enough to trigger cross-reactivity by a process called molecular mimicry. The immune system under stress might make such an error in genetically vulnerable children.
Once triggered, antibodies, cell-mediated immunity, or both could deal the blows. Some investigators favor autoantibodies as the direct or sole cause of the problem. Several different autoantibodies have been found in blood or spinal fluid of children with opsoclonus-myoclonus. Most are IgG; others are IgM. However, they are not always found and some occur in normal persons.
We have proposed that T cells and cytokines may be instrumental to the autoimmune injury found in pediatric opsoclonus-myoclonus. Even if autoantibodies are involved, their production may require the collaboration of T cells. Our testable hypotheses of opsoclonus-myoclonus are diagrammed in the articles referenced below and our research program focuses on this issue.

How can opsoclonus-myoclonus be stopped?
An immunotherapy that helps one disease may not help another. The reason for differences in response has to do with the type of underlying immune problem. Some approaches are directed at a specific cell type, while others are aimed at re-regulating the system through antibodies against autoantibodies (anti-idiotype antibodies). Most treatments that target lymphocytes are more effective against T cells than B cells. Current therapies have only limited ability to remove antibodies that have already entered the brain and bound to target cells. Specifics of treatment options can be found through the menu on the home page.
The use of multiple agents that work by different mechanisms has distinct advantages, especially since the underlying abnormality in opsoclonus-myoclonus has not yet been identified. Some invasive approaches such as thymectomy or bone marrow transplantation have not been explored because there is not enough data to justify their invasiveness and the risks they pose. An important advance would be in being able to predict which child needs aggressive measures.
Other therapies in development offer novel solutions. Why not use the body's own non-toxic resources to fight off the disease? We need to selectively hone on only those white blood cells causing disease, not shut down the whole immune system. Research on opsoclonus-myoclonus is essential for finding out the cause and devising better, more specific treatments.
For a detailed review and references, see:
Pranzatelli MR, Paraneoplastic syndromes: An unsolved murder. Seminars in Child Neurology 7(2):118-130, 2000 [87 references].
Pranzatelli MR, The immunopharmacology of the opsoclonus-myoclonus syndrome. Clinical Neuropharmacology 19(1): 1-47, 1996 [285 references].